FAQs – Copper Levels
Posted April 15, 2016 by intecamericaCategories: pools
Tags: copper ionization, Copper ionizer, Open pool, pools, swimming pool, Testing Copper Levels
CHLORINE ALTERNATIVE FOR SWIMMING POOLS
Posted March 4, 2015 by intecamericaCategories: Uncategorized
Most of you are already familiar with the types of chlorine used in swimming pools (calcium hypochlorite, sodium hypochlorite (bleach), lithium hypochlorite, dichlor, and trichlor). The first three are not stabilized. Meaning once exposed to the sunlight, it will quickly precipitate out of the pool. This is why dichlor and trichlor have become so popular since the stabilizer is added into the dry chlorine.
You are also probably familiar with a “salt pool,” or a salt water generator (SWG) which converts sodium chloride salt into chlorine. Yes, salt water pools are chlorinated pools. When the water chemistry is properly maintained, SWGs can drastically reduce the need to shock your swimming pool. The downside is that overtime, SWG pools can be corrosive to the coping and decking. If you have a vinyl liner pool, there is a chance that the frames can corrode which is a very expensive fix.
Today’s consumer is more sophisticated than that of the previous generation. There is also a growing segment that is concerned with the toxins in our environment and are living healthier lifestyles. Many suffer from chlorine allergies or being hypertensive to chlorine. There is also a decent amount of evidence on increased asthma in young athletes which are involved with competitive swimming in indoor chlorinated pool. There are just a few of the reasons why more consumers are seeking a chlorine alternative.
CHEMICAL OPTIONS
Bromine is in the same halogen family as chlorine. It does have its advantages and disadvantages. Unlike chlorine, it cannot be stabilized with a product such as cyanuric acid. This is a problem with outdoor pools since the sun will quickly burn off the bromine. Bromine would need to be constantly added which would become costly. Now is does have advantages over chlorine for indoor pools. Bromine with not produce a byproduct like chloramines. Chloramines are very irritating to the eyes and can cause a strong odor. Chloramines has also been linked to an increase in asthma for swimming athletes.
Baquacil, Aqua Silk, and Softswim use polyhexamethylene biguanide (PHMB) as the active ingredient.
The main advantage PHMB has over chlorine is it is not an oxidizer and it is not significantly affected by sunlight. Therefore, PHMB residuals are much more stable in both pools and spas than chlorine. The disadvantage is that it is not an oxidizer and a 27% hydrogen peroxide shock treatment is necessary for clarity. These products do not irritate the eyes or skin. These products are by far the most expensive choice in maintaining your swimming pool. Expect to pay more than double to maintain biguanide pools.
PristineBlue has an active ingredient of copper sulfate pentahydrate. Copper is a very effective algaecide. Like the aforementioned products, it is not an oxidizer so you will need periodic shock. The problem is that anything that you need to maintain the pool, you should stick with its complementary and proprietary products. This can get expensive but perhaps not quite as expensive as biguanide based products. Not all stores carry this product so one may spend a lot on gas trying to locate it.
NON-CHEMICAL OPTIONS
Non-chemical treatment would include ionization (copper, copper/silver, copper/zinc), mineralizers (Pool Frog, Nature 2), ozonation, and U/V Light. The last 3 items do not provide a “residual” for sanitation and the biocide component is short lived making it a poor choice as a primary sanitizer. Do not get me wrong, they do offer wonderful benefits. They all reduce the amount of chlorine that is necessary to maintain a pool. Ozone and U/V are becoming more popular with the combined use of chlorine to reduce the disinfection byproducts (DBP such as chloramines and thihalomethanes) and can reduce the frequency of utilizing shock treatments. Of these non-chemical alternatives, only ionization has a long lasting disinfection which makes it the most attractive option. Ionization can be used with anything listed above including chlorine or be used in a “chlorine free” pool.
Ozone is a very strong oxidizer and is used for many applications. Intec has used ozone quite a bit for treating high levels of iron, iron bacteria, as well as hydrogen sulfide. I traditionally have not been a huge fan of ozone for swimming pools. It has been quite expensive and did not offer the benefits I was seeking. It can reduce chlorine levels required for swimming pools and is effective a reducing chloramines which also reduces how often pone might need to shock a pool. However, the new DEL Ozone Solar Eclipse has changed my mind. The new plasma gap technology that produced hydroxyl radicals and ozone. This is a beast. It is pricey but it is the only ozone system that should be considered for pool sanitation. Everything else is just a toy.
UV or ultraviolet light is a concept I still do not see how it made it into the swimming pool market. I do like UV light being used in a closed loop system such as point-of-entry for a home. I have sold quite a few of them. The sanitation takes place inside the quartz sleeve (a clear tube water flows through running parallel with the UV bulb). So if a pool recirculates every eight hours, you have once change to get the bacteria, virus, or algae spore. Bacteria and algae do replicate a lot faster than this. It is also being marketed as reducing chloramines. This I can understand to a point. However, calcium will scale over the quartz sleeve making it not as effective. It is just another widget being sold so more money can be made.
Ionization has been around for a couple of decades and there has been quite a bit of advancement. Ionization is the process in which a current is supplied to a sacrificial electrode made of various metals and subatomic particles, ions, are pulled off and pass through a stream of running water. The composition of metals include copper, copper/silver, or copper/zinc. These metals are toxic to many form of bacteria and algae. At recommended levels, it is totally safe for humans and animals. It is important that only high purity metals are being utilized. Otherwise impurities such as carbon can stain a pool. It is also important to go with a company that has been around a while. Many have entered the market place and have gone in just a couple of years which is a nightmare for warranty or replacement parts such as electrodes. If they have not been in business for at least 10 years, do not even consider them regardless of their technology or sales pitch. Also make sure you evaluate the replacement costs of the electrode. There are a few companies that offer $300 ionizers that have a replace electrode for $170. The life is only 3 months. That is an expensive system for owning a pool. Other systems may be more expensive upfront cost but have electrodes that lasts 2 or more years. The small systems or the solar units to not have enough power to treat a 20,000 gallon pool for example despite their claims. It does little good if one has to run an ionizer 8 hours a day every day to maintain copper levels. If algae is present, it would not have the power output to overcome the algae and supply an acceptable residual of ionic copper.
Intec is a manufacturer of premium ionizers and utilizes only high purity copper for its electrodes. You can place your trust in our 40 plus years of experience with the ionization technology.
Swimming Pools and Turtles – Yes We Handle Anything/Everything
Posted June 6, 2014 by intecamericaCategories: Uncategorized
Tags: cloudy water, nitrates, phosphates, sanitation, swimming pools, turtles
I had the best conversation yesterday with a fascinating woman. Her story is so unique, I had to share. I am an animal lover like Bonnie and when I was little I did catch a lot of turtles. She is not our client and does not have one of our ionization systems However, I did consult with her on how to best handle her pool chemistry with her pets being present. Enjoy…..
Thank you Steve, for the great advice on how to survive my current predicament! The moral of the story, before I even attempt to tell the story, is that turtles and swimming pools are not a smart mix. But that is what I am currently stuck with. Turtles are literally algae fertilizer producers so instead of a swimming pool, I have a 30,000 gallon turtle aquarium which people love to swim in. (BUT, it is STILL cleaner than swimming in a lake.)
I understood that turtles and swimming pools were not going to be a smart mix, but my husband refused to listen and insisted they stay in the pool after they kept escaping from the small pond I built for them. (One literally tried to climb the 18 inch high, chicken wire fence I put around the pond area.) The fact this same turtle decided to crawl up his leg and sit on his lap, as he was relaxing in his floating lounge chair, was what contributed to his making that fatal decision. Suddenly, the turtles stopped being just my turtles and became our turtles. It then took the swimming pool turning green and him spending a fortune on chlorine, plus a few fights with me and me doing the research, for him to finally realize the need to start adding a phosphate remover on an annual basis.
Ten years have now past and my husband sadly pass away. And now I am left with this major problem. My husband had done all the pool maintenance and I decided to hire a company to help rather than deal with the pool as well as learning to survive in other house hold issues without his help. (Even finding a pool maintenance company, that was WILLING to take care of a pool with turtles in it, proved to be a challenge.) I did investigate the possibility of relocating my turtles, but I have REALLY happy turtles, that love their habitat, and love it when people come and swim/play with them. And I really do love my turtles (they are soooo much fun to watch!). So relocating them back to a small residential aquarium is not really an option, nor is releasing them into the wild; that would just kill them. (And forget all you out there who may just want to make turtle soup.)
Bottom line, I am in the process of trying to figure out how to continue to maintain a clean swimming pool while I also pursue finding them an even better living environment. (Anyone know of the existence of an aquarium petting zoo out there??) I realize I will eventually have to relocate them or I will have no choice but to leave them this house in my will. They will, if they continue to stay healthy, out live me. And that is how I ended up in a phone conversation with Steve. My swimming pool ended up turning green because the pool maintenance company did not use the phosphate remover before it was too late. So I decided to do some more internet investigation on how to properly handle having turtles in my swimming pool. Trust me, I have discovered there are very few out there as crazy as I am to have turtles in their swimming pool, let alone someone willing to converse with a crazy lady with turtles in her swimming pool. It was a COMPLETE blessing to finally find someone who understood pool chemicals, PLUS how to best deal with nitrates, who was willing to have a crazy conversation with me. I can not thank you enough for reassuring me how Phos Free, which my husband started using, will be sufficient for the time being until I can afford to make changes to my current pool sanitization and filtration system. Thank you again Steve. Have a great life! Bonnie (Fresno, CA)
Phosphates, Food for Algae, Food for Thought
Posted June 5, 2014 by intecamericaCategories: Uncategorized
Tags: cloudy water, green water, phosphates and swimming pools, slimy surface, swimming pools
Phosphate became a household word in the 1970’s when people started to use low-phosphate and phosphate-free laundry detergents. This was to protect lakes, streams, wetlands and other runoff areas from the detrimental effects of excess phosphate. One of these effects is unwanted algae blooms. What’s true for lakes is also true for swimming pools.
Phosphates are derived from phosphorous, the 11th most abundant mineral in the earth’s crust. It makes its way into pool and spa water from a variety of sources, including fertilizers, swimmer waste (sweat and urine), decaying vegetation, cosmetic items, detergents, and even tap water of many cities (which contains compounds used to treat corrosion). Phosphates can also be found in many pool products such as stain removal products. . Phosphate is persistent and does not break down naturally.
Phosphates are a food source for all strains of algae and can make controlling their growth difficult. Not removing the phosphates will cost you more time and money on maintenance. Remove the food, and you have a strong weapon against algae.
When excess phosphate is present in a swimming pool, the symptoms often include the following:
- Cloudy, Green Water
- Slippery and Slimy Surfaces
- Mustard and Green Colored Debris, especially on automated cleaners or hoses
- Excessive Chemical Consumption
- Poor Water Quality
- For ionization systems, difficulty in getting the copper level up to desired levels.
- For chlorination, increase the amount of chlorine required to keep algae in check.
It is debated about the appropriate phosphate levels. From my research it appears 50 ppb (Note-not 50ppm) or less is ideal. You can maintain a pool with levels as high as 1,000 ppb, but it appears that more money is being spent on maintenance even at levels as low as 300 ppb.
There are many products available on the market to remove phosphates. Phos-out and PhosFree are two popular products that have excellent feedback results. For areas that are prone to phosphates, there is a weekly maintenance product called Pool Perfect by Natural Chemistry. These products work by locking up the phosphates into a suspended solid that is filtered out by your filter. As the filter clogs with the suspended solid, backwashing sends the former phosphates into the street.
Regular pool maintenance:
The preventative measure of limiting phosphate exposure is taken by doing the following:
Remove leaves and organic material from the water as soon as possible
- Vacuum and clean filters and pump baskets regularly
- Be aware of the phosphate content of cleaners and chemicals used in and around the swimming pool
- Don’t allow drainage from plants or the lawn to enter the pool
Swimmin Pool Water Level
Posted May 14, 2014 by intecamericaCategories: pools
Tags: Pool Maintenance, Pool Water Level
The level at which the swimming pool skimmers operate best is between one third and one half the way up the opening of the pool skimmer. If the level is higher, the water moving into the skimmer is going so slow that debris may pass by the opening without being pulled in. If the pool water is so high that it covers the skimmer opening, floating debris can’t get in. If the water is too low the skimmer can bottom out, thereby sucking air into the system which can result in losing the prime and possibly result in burning up your swim pool filter pump motor. Add water before backwashing and vacuuming the pool because this will also lower the water level. source – http://www.swimmingpool.com/maintenance/general-maintenance-and-tips/pool-water-level
Posted May 9, 2014 by intecamerica
Categories: Uncategorized
Tags: Acid Water, Alkalinity, Basic Water, pH, Ph and alkalinity relationship, swimming pool
As most of you know, we have been treating water for over 40 years. Over those years, we have come across some really amazing scenarios that are not in any text books or training materials. From treating poultry water to paper mills, private wells to surface waters, and of course swimming pools. We truly have experienced it all. The one question that stands out the most is pH and the relationship to alkalinity.
pH is the single most misunderstood/overlooked variable in water treatment. It is also the single most important. Whether you are removing manganese from a water well or trying to clear up a swimming pool, you MUST have to consider pH first.
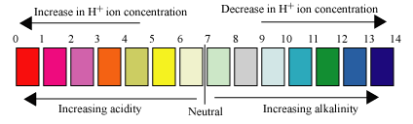
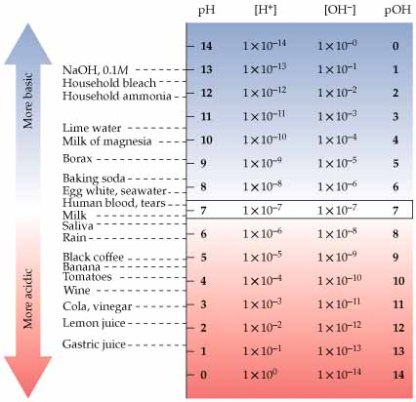
pH and alkalinity are two different measurable parameters of water. pH is a measure of the acidity or basicity of an aqueous solution. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline. Alkalinity is a measure of the buffering capacity of water. Or simply its ability to resist sudden changes in pH.
pH Concentrations Scale
What does the lower case “p” and the capitol “H” stand for? In the United States, the “p” normally stands for “potential” and “H” stands for “Hydrogen”. Therefore, pH stands for the potential of Hydrogen. It is the hydrogen ion that determines the level of acidity or alkalinity.
An example of a weak acid is acetic acid (vinegar), which releases only small amounts of free hydrogen ions into solution. Sulfuric acid is considered a strong acid because it releases more hydrogen ions into solution. Highly acidic water can be corrosive. Note – all acids have an “H” in the chemical formula.
An alkali can be defined as a substance that releases hydroxyl ions when dissolved in a solution. The higher the concentration of the hydroxyl ions in a solution, the higher the resulting pH will be. Highly alkaline waters can dry out the skin. Common alkalis include hydroxide, carbonate, and bicarbonate salts such as calcium, magnesium, potassium, and sodium. Note – All alkali have “OH” in the chemical formula.
Acid (H+) + Alkali (OH–) = H2O
Application
If your pH is over 7.0, your water is called alkaline. You have too much OH– floating around. When you add muriatic acid for example, H+ will combine with OH– and produce H2O and reduce your alkalinity and also reduce your pH.
If your pH is below 7.0, your water is acidic and contains too much H+. Your alkalinity is probably too low in this situation. When you add baking soda (sodium bicarbonate), the OH– will neutralize the excess H+ to form H2O. It will raise the alkalinity and raise the pH also.
Defined, alkalinity is the buffering agent that maintains/stabilizes pH. In your pool, it dictates how fast your pH will rise. This will vary by regions depending on the other dissolved solids in your water. For most areas of the country, your pH will balance at 7.2 with an alkalinity of 60-80. Keep in mind, this is a rule of thumb, and NOT the law.
You do not need to test for alkalinity often. But when you are fighting your pH, it must be tested and noted in a journal. If you are opening your pool and adding acid more than once a week, that is a great time to do an alkalinity test. High alkalinity will drive pH up. The higher the alkalinity, the faster your pH will rise
I will give you an example. If you and your neighbor have a pool (same size, same surface, same bather usage) and you have an alkalinity of 200 and your neighbor has an alkalinity of 100, then your pH will rise twice as fast as theirs because your alkalinity is twice as high (again, just an rule of thumb and not scientific law).
Let’s say your pH is 7.8 and alkalinity is 200. When you run your acid demand test and add the appropriate amount of acid, your pH may drop to 7.0 and your alkalinity may drop to 180. Your pH will rise again and possibly be out of range in 2 days. When you treat your 7.8 pH again, your alkalinity may drop to 160. But instead of 2 days, it is 3 days before it reaches 7.8. Each time you treat your pool with an acid, your alkalinity with drop. Perhaps on the 4th or 5th treatment, your alkalinity will be 80 and remain in balance for 7 days without going over range (which is a maximum of 7.4 for ionization). That is the sweet spot you are looking for.
Keep in mind this is merely an example. You will have evaporation, splash out, make-up water, and rain. Your pH will NEVER remain constant. There are some automated systems than can measure pH and automatically add acid to your pool for a more hands free approach. But maintaining pH is an ongoing effort and this is where you spend most of your time.
For an ionized pool, we do recommend testing twice a week. The average person treats their pool by adding and acid or base once a week. For those that do nothing, you are just lucky. But we do have a few of those out there.
If this all sounds confusing, that is OK. We have a toll free number to assist you with this and any other questions you may have regarding your swimming pool.
Chlorine Alternative for Swimming Pools
Posted April 18, 2014 by intecamericaCategories: Uncategorized
Tags: Aqua Silk, Baquacil, biguanide, chemical free pool, Chlorine alternative, chlorine free pool, copper ionization, copper/silver ionization, ozone for swimming pools, PHMB, pool ionizer, pool water chemistry, Pristine Blue, Softswim
Chlorine Products Used Today
Most of you are already familiar with the types of chlorine used in swimming pools (calcium hypochlorite, sodium hypochlorite (bleach), lithium hypochlorite, dichlor, and trichlor). The first three are not stabilized. Meaning once exposed to the sunlight, it will quickly precipitate out of the pool and/or be converted to chlorides. This is why dichlor and trichlor have become so popular since the stabilizer is added into the dry chlorine.
You are also probably familiar with a “salt pool,” or a salt water generator (SWG) which converts sodium chloride salt into chlorine. I am still amazed of many people still do not understand that salt water pools are chlorinated pools. It simply converts chlorides into chlorine (hypochlorous acid and hypochlorite ions when in water). When the water chemistry is properly maintained, SWGs can drastically reduce the need to shock your swimming pool. The downside is that overtime, SWG pools can be corrosive to the coping and decking. If you have a vinyl liner pool, there is a chance that the frames behind the liner will corrode which is a very expensive fix. Do not be fooled, since it is a chlorinated pool, you will still have the same disinfection byproducts as a traditionally chlorinated pool.
There is a growing segment of the population that is concerned with the toxins in our environment and are living healthier lifestyles. There is a debate going on now if there is such a thing as chlorine allergies (I will leave that up to the medical doctors). However, there is a general consensus that a small percentage of people are actually hypertensive to chlorine. There is also a decent amount of evidence on increased asthma in young athletes which are involved with competitive swimming in indoor chlorinated pool. Only time will tell, but consumers are not wanting to wait. This is why more consumers are seeking a chlorine alternative.
CHEMICAL OPTIONS
Bromine is in the same halogen family as chlorine. It does have its advantages and disadvantages. Unlike chlorine, it cannot be stabilized with a product such as cyanuric acid. This is a problem with outdoor pools since the sun will quickly burn off the bromine. Bromine would need to be constantly added which would become costly. Now is does have advantages over chlorine for indoor pools. Bromine with not produce a gaseous byproduct like chloramines which are very irritating to the eyes and can cause a strong odor. Chloramines has also been linked to an increase in asthma for swimming athletes. The byproducts of bromine and its effects are not fully known at this time.
Baquacil, Aqua Silk, and Softswim use polyhexamethylene biguanide (PHMB) as the active ingredient. The main advantage PHMB has over chlorine is it is not an oxidizer and it is not significantly affected by sunlight. Therefore, PHMB residuals are much more stable in both pools and spas than chlorine. The disadvantage is that it is not an oxidizer and a 27% hydrogen peroxide shock treatment is necessary for clarity. These products do not irritate the eyes or skin. These products are by far the most expensive choice in maintaining your swimming pool. Expect to pay more than double to maintain biguanide pools.
PristineBlue has an active ingredient of copper sulfate pentahydrate. Copper is a very effective algaecide. Like the aforementioned products, it is not an oxidizer so you will need periodic shock. The problem is that anything that you need to maintain the pool, you should stick with its complementary and proprietary products. This can get expensive but perhaps not quite as expensive as biguanide based products. Not all stores carry this product so one may spend a lot on gas trying to locate it.
NON-CHEMICAL OPTIONS
Non-chemical pool treatments would include pool ionizers (copper, copper/silver, copper/zinc), mineralizers (Pool Frog, Nature 2), ozonation, and U/V Light. Mineralizers, ozone, and UV lights do not provide a “residual” for sanitation and the biocide component is short lived making it a poor choice as a primary sanitizer. Do not get me wrong, they do offer wonderful benefits. They all reduce the amount of chlorine that is necessary to maintain a pool. Ozone and U/V are becoming more popular with the combined use of chlorine to reduce the disinfection byproducts (DBP such as chloramines) and can reduce the frequency of using shock treatments. Of these non-chemical alternatives, only ionization has a long lasting disinfection which makes it the most attractive option. Ionization can be used with anything listed above including chlorine. If pool ionizers are size and the water chemistry is maintained properly, then you can have a “chlorine free” pool.
Ozone is a very strong oxidizer and is used for many applications. Intec has used ozone quite a bit for treating high levels of iron, iron bacteria, as well as hydrogen sulfide. I traditionally have not been a huge fan of ozone for swimming pools. It is quite expensive and does not offer all the benefits I am seeking. It can reduce chlorine levels required for swimming pools and is effective a reducing chloramines which also reduces how often pone might need to shock a pool. Ozone still cannot sanitize a pool on its own, but when combined with copper ionization it can be a great clarifier and offer a multi-barrier sanitation approach. The best technology available for the copper ionization and ozonation approach is the Solar Eclipse from EL Ozone which offers a mixed oxidant though is AOP technology. The MSRP on these units is $2,300.
UV or ultraviolet light is a concept I still do not see how it made it into the swimming pool market. I do like UV light being used in a closed loop system such as point-of-entry for a home. I have sold quite a few of them. The sanitation takes place inside the quartz sleeve (a clear tube water flows through running parallel with the UV bulb). So if a pool recirculates every eight hours, you have once chance to get the bacteria, virus, or algae spore. Bacteria and algae do replicate a lot faster than this (algae can double itself every 15 minutes). UV light can convert chloramines to chlorides and I can understand this advantage to a point. However, calcium will scale over the quartz sleeve making it not as effective after a few weeks. It is just another widget being sold so more money can be made.
Copper ionizers is the best chlorine alternative on the market today. Ionization has been around for a couple of decades and there has been quite a bit of advancement. Ionization is the process in which a current is supplied to a sacrificial electrode made of various metals and subatomic particles (ions) are pulled off as water passes though the electrolytic cell. The composition of metals/alloys include copper, copper/silver, or copper/zinc. These metals are toxic to many forms of bacteria and algae. At recommended levels, it is totally safe for humans and animals. It is important that only high purity metals are being utilized in the electrode. Otherwise, impurities such as carbon can stain a pool black. It is also important to go with a company that has been around a while. Many manufacturers have entered the market place and left in just a couple of years. This is a nightmare for warranty or replacement parts such as electrodes. If manufacturers have not been in business for at least 10 years, do not even consider them regardless of their technology or sales pitch. Also make sure you evaluate the replacement costs of the electrode. There are a few companies that offer $300 ionizers that have a replace electrodes for $170. Most are very small and the life is only 3 months. If you have to replace these several times a year, it becomes very expensive. More advanced systems may be more expensive upfront cost but have electrodes that lasts 2 or more years. These units are also more powerful and can replace chlorine altogether. Smaller systems and solar units to not have enough power to treat a 15,000 gallon pool for example despite their claims. What good it is it you have to run it an ionizer 8 hours a day every day to maintain copper levels. If you have algae present, it will never keep up. These cheap systems have given the ionization a back eyes on its reputation.
If you have any questions on the alternative available, please contact your Intec rep today at 800-896-1759 or visit us Intec on the web at http://www.Intec-America.com
Unintentional Drownings: How to Protect Your Family
Posted April 18, 2014 by intecamericaCategories: Uncategorized
Tags: Alarms for Pools, Drowning, Pool alarms, prevent drowning, Swimming Pool Alarms
Every day, about ten people die from unintentional drowning. Of these, two are children aged 14 or younger. This article will offer some tips on reducing risks and help keep your family and friends safe
How big is the problem?
- From 2005-2009, there were an average of 3,533 fatal unintentional drownings (non-boating related) annually in the United States — about ten deaths per day.
- About one in five people who die from drowning are children 14 and younger. For every child who dies from drowning, another five receive emergency department care for nonfatal submersion injuries.Children ages 1 to 4 have the highest drowning rates.
- Among children ages 1 to 4, most drownings occur in home swimming pools.
- Drowning is responsible for more deaths among children 1-4 than any other cause except congenital anomalies (birth defects).
- Among those 1-14, fatal drowning remains the second-leading cause of unintentional injury-related death behind motor vehicle crashes.
What factors influence drowning risk?
- Lack of Swimming Ability: Research has shown that participation in formal swimming lessons can reduce the risk of drowning among children aged 1 to 4 years.
- Lack of Barriers: Barriers, such as pool fencing, prevent young children from gaining access to the pool area without caregivers’ awareness. A four-sided isolation fence (separating the pool area from the house and yard) reduces a child’s risk of drowning 83% compared to three-sided property-line fencing.
- Lack of Close Supervision: Drowning can happen quickly and quietly anywhere there is water (such as bathtubs, swimming pools, and fountains), and even in the presence of lifeguards.
Tips to help you stay safe in the water
- Supervise When in or Around Water. Designate a responsible adult to watch young children swimming or playing in or around water. Supervisors of preschool children should provide “touch supervision”, be close enough to reach the child at all times.
- Learn to Swim. Formal swimming lessons can protect young children from drowning. However, even when children have had formal swimming lessons, constant, careful supervision when children are in the water, and barriers, such as pool fencing to prevent unsupervised access, are still important.
- Learn Cardiopulmonary Resuscitation (CPR). In the time it takes for paramedics to arrive, your CPR skills could save someone’s life.
- Air-Filled or Foam Toys are not safety devices. Don’t use air-filled or foam toys, such as “water wings”, “noodles”, or inner-tubes, to replace floaties for small children. These toys are not life jackets and are not designed to keep swimmers safe.
If you have a swimming pool at home:
- Install Four-Sided Fencing. Install a four-sided pool fence that completely separates the pool area from the house and yard. The fence should be at least 4 feet high. Use self-closing and self-latching gates that open outward with latches that are out of reach of children. Also, consider additional barriers such as automatic door locks and alarms to prevent access or alert you if someone enters the pool area.
- Clear the Pool and Deck of Toys. Remove floats, balls and other toys from the pool and surrounding area immediately after use so children are not tempted to enter the pool area unsupervised.
- Install a Pool Alarm. Now pool alarms can since when a small child falls into a pool. Unlike previous technologies, the new products can detect underwater movement which would prevent false alarms. A good pool alarm would is less than $600 and is a great piece of mind.
For more information on swimming pool alarms, contact you Intec representative at 800-896-1759
How to Get Rid of Water Bugs
Posted April 17, 2014 by intecamericaCategories: pools, Uncategorized
Tags: backswimmer, swimming pool, water boatman, water boatmen, water bug, waterbugs
Two of the most common bugs in your pool are the backswimmer and water boatman. These pests are in the aquatic insect classified under the order Hemiptera. The bugs generally are not harmful to humans, although the backswimmer in particular can deliver a painful bite. Although most bugs feed on algae, the backswimmer feeds on the water boatman as well as other bugs. None of the bugs can live outside of the water for long periods of time, so getting rid of their food supply and nesting places stops bugs from living in the pool.
To get rid of water bugs; attack their way of living. Chlorine and shock treatments are not effective at killing them or controlling their population. In order to eliminate them, one must better understand them and their preferred environments.
Water boatmen: are oval shaped bugs that do not bite, There food source is algae and minute aquatic organisms, they eat mosquito larvae and tend to eat small aquatic animals.
Backswimmers: have a streamlined body shape and DO bite (as painful as a horsefly), the backswimmer will come to the surface for air, a supply of which they carry down with them under their wings and between the fine hairs covering the underside of the body.
- both water boatmen and backswimmers can fly.
- both lay eggs in underwater vegetation, in a swim pools case, “algae”
- both can be found in mud at the bottom of streams, pools and ponds.
- both NEED to come up for air, if you hold them down under water they will drown
- both for pools, lay eggs in the algae.
- Water boatman swim right-side up; back swimmers swim upside down
The only way to get rid of them is to take away their food supply, for water boatmen its algae, for backswimmers its other water bugs such as water boatmen or water beetles. The first step is to balance your water chemistry of course, scrub the algae off of the sides and bottoms of pool, then add bleach or hydrogen peroxide to oxidize the algae. With no algae in the pool, the bugs cannot and will not lay their eggs!
If you live in an area with irrigation or standing water areas, water bugs can migrate. Not only can they fly, they can crawl. By adding Borax around the pool decking, it reduce the change of the bugs crawling from a ditch or puddle into your swimming pool. But remember, they can fly. This is why proper balance of water chemistry is important. When they come to your pool and see there’s nothing to eat, they will leave.
If your pool does get away from you and you find waterbugs in your pool, then here is a simple treatment. At night be sure to turn off all exterior lights surrounding your pool. Put a spotlight at the deep end shining into your pool. Add 2 or 3 teaspoons of liquid dish soap into the pool where the spotlight is shining. The waterbugs will come up for air and be drawn to the light. With the soap on the surface, they cannot penetrate the surface and will drown. Just scoop them up the next morning.
I have read in some forums BioGuard brand Back-Up algaecide tends to work well and is fast acting. This is not a pesticide by any means. However, there are reports of buds dying off 24 hours after treatment.
Recap – being proactive and maintaining your water chemistry is the most effective way to keep water bus out of your pool. When a population is established, one can use a liquid dish soap treatment or the Back-Up algaecide.
Opening your Swimming Pool
Posted April 17, 2014 by intecamericaCategories: Uncategorized
Tags: How to open your pool, Open pool, opening a swimming pool, pool cleaning, pool opening, swimming pool
It is almost that time of year again. Time for backyard bar-b-queues, parties and swimming. Follow these steps to ensure you are off to a great start to your swim season.
- Remove the cover if you have one. Make sure to get rid of any debris that may be on the cover.
- Inspect pump, plumbing and filter for cracked or damaged parts.
- Reconnect all plumbing (if you disconnected when you closed the pool).
- Remove any winter plugs you may have used and reconnect eyeball jets to the returns.
- Remove any debris from skimmers and weir basket.
- Remove any debris from pool floor with rake or leaf skimmer.
- Fill pool so that the water is 3” to 4” above skimmers.
- If you are using a cartridge filter, make sure filter is clean.
- If you are using a sand filter, make sure sand is in good shape, should be replaced every 5 years or so.
- Make sure valves are set to recirculate the water and turn on the pump.
- Check and balance your chemical levels: pH, Total Alkalinity, Calcium Hardness, and Copper or chlorine. Refer to operator manuals for suggested ranges.
- Once your pool water is clear, the pool itself is clear of debris and the chemistry is in balance, dive in and enjoy!
If you have any questions on opening your pool or copper ionization, please call Intec America at 1-800-896-1759.